Our response to the GOC call for evidence on the Opticians Act 1989 and consultation on associated policies
Our submission to the consultation, July 2022
The General Optical Council (GOC) launched its call for evidence on the Opticians Act and consultation on associated policies on 28 March. This could eventually result in some of the most significant changes to regulation for the optical sector in years. The call for evidence ran for 16 weeks, closing to submissions on 18 July.
Below is our response in full, covering eight sections. Throughout this submission, we have predicated our response on maintaining consistently high standards of patient safety and care as well as the definitions contained within the current legislation and guidance.
The AOP is proud to be the leading representative membership organisation for optometrists in the UK, supporting over 82% of practising optometrists on the GOC’s register. Our response has been co-produced through extensive member, sector and stakeholder engagement and communications activity including:
- Member surveys (see below)
- Workshops (AOP Board, Council)
- Committees (AOP Policy Committee, Virtual Policy Group, and Forums)
- Sector body meetings (regular sessions to explore common principles and socialise thinking)
- UK country engagement (joint meetings between the sector bodies and UK country organisations)
- Social media (a range of interactive social media activity including Instagram, Facebook, and LinkedIn posts)
- Website articles
- Content in Optometry Today
- Press releases from the AOP
As a membership organisation, it is not surprising that member engagement formed a key part of our work on the consultation. As part of this we conducted a survey of registrants. The survey yielded 2,445 responses, the largest number of any that the AOP has conducted in recent times.
We carried out a survey of our members in which we asked about four key areas of the GOC’s consultation:
i. The separation of the refraction and eye examination elements of the sight test
ii. The delegation of elements of the sight test to other professionals such as dispensing opticians
iii. The requirement to verify a contact lens prescription with the original prescriber
iv. The requirement to have a prescription less than two years old before contact lenses are fitted/re-fitted
We have signalled registrants response to these questions throughout the document.
We would like to express our thanks to all those who took part in our sector engagement programme, the insight from which has helped to inform our submission to the GOC.
Contact our Policy team if you have any questions: [email protected]
Section 1: GOC objectives for legislative reform (Para 14-15)
Q5. Are these the right objectives for the GOC for legislative reform?
AOP response: No
The principles set out here are generally sensible and fit in the broad scope of the wider Department of Health and Social Care (DHSC) consultation on regulation and opportunities for regulatory reform across health care. However, there are some inconsistencies and issues within the principles of the call for evidence and consultation which need to be addressed before any subsequent work is undertaken. We also think that there are additional points that the GOC should consider in ‘next steps’ planning as set out below.
The GOC has previously indicated that it will consult before any subsequent business case for change to the Opticians Act. We believe the GOC should reaffirm this by explicitly stating that it will conduct a full public consultation before developing a case for change to the DHSC. This should also include a full impact assessment of potential changes, and the costs and benefits of different options. This is essential because of the range, scope and importance of the changes being considered and their potential impacts on public protection and patient safety. Further consultation and assessment of impacts and options should help refine the case for change and make it more targeted, proportionate and effective.
It would also be helpful for the GOC principles to explicitly include reference to working in line with the Professional Standards Authority (PSA) PSA standards of good regulation which are:
- Proportionate
- Consistent
- Targeted
- Transparent
- Accountable
- Agile
Issues to address with the current GOC objectives
The GOC’s introduction to the objectives says that they are non-hierarchical, but objective 1 states that ‘maintaining patient and public safety is the primary objective’. We agree that this is the most important objective, and the AOP’s own response is grounded on its primacy. The GOC should make this clearer.
The objectives should also explain that the burden of proof for any prospective case for the removal of existing legal restrictions should be a robust demonstration that changes to the Act will maintain public protection, and not introduce new risks of harm.
Objective 5 should be reframed to read more simply, for example as ‘ensuring consistency in the whole system of optical regulation, across individual practitioners and optical service providers’.
Objective 7 says that the ‘path of least resistance’ should be taken. This is true when public protection and patient safety can be achieved effectively via different regulatory mechanisms and guidance. However, the GOC should prioritise the most appropriate and proportionate level of regulation required to protect patients and the public from harm. In some cases, this may not be the ‘path of least resistance’, but a safety-focused regulatory model which targets risks of harm and enables a good standard of clinical care from trained and registered clinicians.
Section 2: Protection of title, restricted activities and registers (sections 7, 8A, 9 and 24-30A of the Act)
Q6. What activities should non-registrants be restricted/prevented from doing?
The current restrictions on the activity of non-registrants should remain in place in order to protect the public and patients from harm.
An evaluation of the public’s perceptions shows that sight is the most highly valued sense.1 We can infer that the public would expect that clinical procedures relating to sight should only be carried out by appropriately trained and accountable clinicians. In order to protect the public, it is our view that non registrants should be restricted from undertaking the testing of sight and the fitting of contact lenses.
We also think that the legal restrictions on supply of optical appliances from non-registrants should remain in place, and be more effectively enforced by the GOC, including the supply of optical appliances for those patients classed as ‘vulnerable’ (as defined in the current legislation). We have explained and evidenced this in more detail in our answers to: Section 2, Question 7; and Section 6.
References
- Enoch, J., McDonald, L., Jones, L., Jones, P. R., & Crabb, D. P. Evaluating whether sight is the most valued sense [published online October 3, 2019]. JAMA Ophthalmol
Q7. What activities do you think must be restricted to our registrants?
The current list of legal restrictions on activities that can only be performed by registrants should remain in place in order to protect the public.
It is our opinion that particular patient groups should only be supplied with optical appliances by, or under the supervision of a registered optometrist, or dispensing optician (see answers in Section 6).
This is because the practice of ensuring that the correct appliance is provided can be more difficult because of the patient’s additional needs, for example a patient with dementia, a patient with a learning disability, a patient with a physical disability or a patient with additional sensory impairment. It is also important to note that some patients are more pre-disposed to sight problems. For example, patients with a learning disability are 10 times more likely to have an eye condition than the general population and the proportion is much higher for people with severe or profound learning disabilities. Restricting the supply of optical appliances by, or under the supervision of, a registered optometrist or dispensing optician would provide much needed additional protection to these patient groups as well as increasing the opportunity for early intervention and diagnosis.2
A good example of how these challenges have been addressed is through the roll out of the NHS England Special School Eye Care Service, following a highly successful early adopter programme.
Whilst we think there is value in expanding the list of vulnerable patients in the interests of better clinical care and patient outcomes, it should not be done if and where it risks compromising existing protected groups.
References
2. Learning Disability and Sight loss.pdf (rnib.org.uk)
Q8. What are your views about continuing to restrict/prevent non-registrants from carrying out the following activities?
a) Testing of sight - AOP response: should be restricted
b Fitting of contact lenses - AOP response: should be restricted
c) Selling optical appliances to children under 16 and those registered visually impaired - AOP response: should be restricted
d) Selling zero powered contact lenses - AOP response: should be restricted
Q9. Are there any additional activities that you think should be restricted to registrants?
Based on consultation with members, we have no strong opinion as to the addition of other activities which should be restricted to registrants. However, we do feel that vulnerable patient groups should only be supplied with optical appliances by, or under the supervision of, a registered optometrist or dispensing optician (see also our response to question 6 and 7).
Q10. Is there any evidence that any other post-registration skills, qualifications or training need to be accredited or approved by the GOC (above and beyond the existing contact lens optician and prescribing qualifications)?
AOP response: No
Mechanisms already exist for professional and inter-professional standards in post-registration training to be created and implemented, for example the professional certificate in glaucoma care. The existing post graduate qualifications are delivered by existing education providers such as universities and assured by the College of Optometrists.
We have spoken to several of our members who are education providers who said that the current educational market already enables new provision to be offered where there is demand for it, and that GOC involvement would unnecessarily complicate the delivery landscape.
We do not think there is evidence that GOC accreditation or approval of additional post-registration qualifications or training is necessary. It is unclear from the call for evidence what kind of process the GOC would use to accredit additional qualifications. We are not aware of other healthcare professional regulators, such as the General Medical Council (GMC), undertaking accreditation of additional post-registration qualifications. Were any such accreditation process to be introduced, it would need to be properly resourced, structured and implemented by the GOC, and we think there would be risks of the process not working properly or creating unintended problems.
Standardising the register
The current register lists GOC-approved qualifications (such as Independent Prescribing and contact lens qualifications) and separately lists non-GOC-accredited qualifications such as professional organisation memberships and other training. This is the correct approach, but it would be helpful for the GOC to review how its register is structured and used so that it can be easily searched and understood. It would also be helpful if the GOC considered how the new powers of register annotation could appropriately be used without the need for additional GOC educational accreditation.
There would be a number of benefits for professionals to doing this, including supporting career progression, recruitment and retention. For patients, an easily searchable register will support better signposting at system, place and neighbourhood level within primary care.
Section 3: Regulation of businesses (sections 9 and 28 of the Act)
Q11. Does the basis for extension of business regulation outlined in our 2013 review of business regulation still apply?
AOP response: Yes
We believe that there is a strong case for extending business regulation. This is because in clinical practice the patient’s experience is determined not only by the individual practitioner, but fundamentally by the environment in which they practice, and the demands put on them by their employer. The adequacy of the clinical environment is determined by the business through its policies, processes, and employment practice.
Many businesses also provide pre-registration placements. The clinical environment, supervision, and the ethical standards of a business can make a big difference to an optometrist at the beginning of their career. The new education system arising from the GOC’s education strategic review will put even more onus on employers to provide students with a quality learning environment throughout their education. Our educationalist members note that students are strongly influenced by their placement environments. Businesses should be accountable for the educational environment they provide.
We believe that business registration should be compulsory and should apply to any business entity carrying out protected functions (sight testing, contact lens fitting and dispensing to restricted groups). This would mean extending registration (and therefore regulation) to businesses owned/run by lay owners as well as all registrant-run businesses. It would also include businesses that carry out protected functions but do not employ UK-registered professionals, over which the GOC currently has no regulatory powers.
To enlarge on the above points, our reasons for supporting comprehensive business regulation are:
- All organisations carrying out protected functions and providing services to members of the public should be obliged to meet standards designed to protect patients/customers. This should include online businesses dispensing contact lenses or spectacles to restricted groups, as well as traditional optical practices
- The policies and practices in businesses create the environment for individual clinicians to succeed or fail. This environment includes the physical space, the equipment, management of the appointment diary, the way time is allocated for record-keeping or training and development, referral arrangement (both internal within the practice and to secondary care), and even pay incentives. All these can contribute to better or worse patient care, experience and outcomes. Individual registrants need to take responsibility for their standards of care, but we strongly believe that businesses should be held to account for their part in creating the care environment, and the practice of non-registrants they employ
- Requiring registration would make it easier for the GOC to investigate and prosecute concerns about any aspect of business standards, such as unsupervised dispensing to children or selling appliances without appropriate verification of an in-date prescription, and would enable the GOC to respond to any future changes in business models that might come about as a result of technology changes.
Q12. Are there any advantages, disadvantages and impacts (both positive and negative) of extending business regulation in addition to those identified in our 2013 review of business regulation? (Impacts can include financial and equality, diversity and inclusion.)
AOP response: Yes
Our view is that the biggest potential disadvantage of compulsory business regulation could be the creation of an unfair and disproportionate financial burden on smaller businesses which are run by registrants. For a registrant practice owner to pay both individual and business registration from a single practice income would clearly be more burdensome than for a non-registrant owner of a larger business. The GOC would need to consider how to avoid this burden. A few possible ways of achieving this could be:
- To make the business registration fee proportionate to practice income, so that larger businesses pay a larger fee
- To charge a nominal business registration fee but impose more significant penalties for breaches of business standards that are proportionate to the actual harm and business size
- To have different fees for the registrant vs non-registrant owned businesses, that take into account that registrant business owners have already paid an individual registration fee
There are some clear advantages (some already mentioned above) but in summary they are:
- Equal application of standards across all organisations carrying out restricted functions
- Businesses will be required to take responsibility for creating an environment in which excellent care can take place
- The GOC will be able to respond more quickly and flexibly to changes in business practices and set-ups, including online optical businesses
Q13. Do you think the GOC could more effectively regulate businesses if it had powers of inspection?
AOP response: Not sure / no opinion
We recognise how giving the GOC inspection powers could allow the GOC to assure itself that businesses are meeting its standards. However, we believe the use of inspection powers would place burdens on the sector that are likely to be disproportionate to the risks posed to the public and patients.
In fact, a burdensome inspection regime could impair the ability of practices to deliver care services. The effectiveness of inspection regimes has been called into question several times in recent years when organisations which passed inspections were nonetheless found to be operating unsafely. Mid Staffordshire NHS hospital trust is the most notable example of this. A recent study published in the BMJ mapped the problems of NHS providers facing multiple and disparate regulatory and inspection regimes, and concluded that the overall impact actually hindered quality and safety initiatives.3 To be effective and avoid unintended detrimental impacts any inspection process would need to:
- Have clearly targeted and risk-based regulatory goals4
- Be proportionate to the levels of risks in optical practice
- Be accountable and transparent in its design and delivery to ensure it is a trusted process
- Avoid unnecessary overlap with NHS regulatory regimes
- Be responsive to feedback from stakeholders to continuously improve design and delivery of the inspection process
- Be agile to new areas of risk in optical practice
We are pessimistic about the possibility of all the above criteria being met. For example, the risks in optical practice are very low, and any inspection regime would need to be very low-cost and light-touch in order to be proportionate to those risks. To run a comprehensive inspection regime would come at significant cost, which would be passed on to registrants. If the GOC did receive inspection powers, it should be required to account for the way registrants’ money is used and to demonstrate that the burden is justified in the light of the risk involved and the benefit to patients and the public.
It is also unlikely that a regime would avoid overlap with other regimes. There is already an assurance framework for many elements of good business practice for those optical businesses that deliver GOS (which is around 95% of practices). These practices are already obliged to demonstrate compliance with the NHS General Ophthalmic Services contractual requirements, which means that they must already show that they have good employment and clinical governance policies and procedures in place. Optical practices, which are generally small organisations, should not be required to participate in duplicate assurance processes. Preparation for a further inspection assessing the same factors would have a disproportionate impact on their ability to operate.
Although we are sceptical about the GOC having inspection powers, we do believe that the GOC should have the power to investigate complaints about specific examples of failure to meet standards. These would be matters brought to the GOC’s attention by concerned members of the public, whistle-blowers or NHS advisers.
References
3. Oikonomou, E., Carthey, J., Macrae, C., & Vincent, C. (2019). Patient safety regulation in the NHS: mapping the regulatory landscape of healthcare. BMJ open, 9(7), e028663
4. Beaussier, A. L., Demeritt, D., Griffiths, A., & Rothstein, H. (2016). Accounting for failure: risk-based regulation and the problems of ensuring healthcare quality in the NHS. Health, risk & society, 18(3-4), 205-224
Q14. Is there an alternative model of business regulation that we should consider?
Answer: Not sure / no opinion - Whilst there are examples of optometrists in large practices taking on a supervisory role with confidence, we believe that the “responsible optometrist” concept could deflect responsibility for quality from the business owners – who set the organisational policies, procedures and culture. This could mean that individual registrants become scapegoats for problems caused by matters beyond their control. At this point in time, we are not clear what alternative model of business regulation would be appropriate for the profession.
Section 4: Testing of sight (sections 24 and 26 of the Act)
Q15. Should dispensing opticians be able to undertake refraction for the purposes of the sight test? (NB This would be possible only if the GOC were to amend or remove its 2013 statement on refraction.)
AOP response: No
Based on our legal advice, provided by Queen’s Counsel, the Opticians Act does not permit delegation of the sight test. The simple removal of the current GOC statement on the subject would not alter the unambiguous legal position created by the Act. Allowing dispensing opticians to refract, in particular without supervision, would create a significant risk of missed pathology which could endanger the nation’s eye health.
The response to our member survey on the call for evidence indicates that the overwhelming majority of optometrist registrants do not see any significant benefit to patients or practices, should this be allowed. In the survey, we asked, “do you think that elements of the sight test could appropriately be delegated to another professional such as a DO?” We had 2449 responses to this question of which 4.6% said that they ‘definitely’ could, with a further 28.9% saying that they could, but ‘only within strict guidelines’. However, 64.41% felt that ‘no elements’ of the sight test could be safely delegated (see figure 1 below).
Figure 1
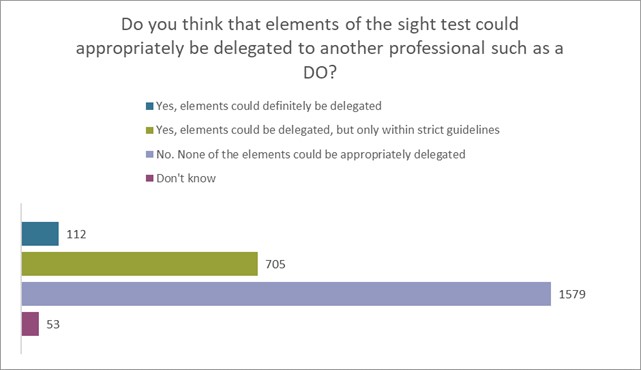
Refraction within the sight test goes beyond the simple identification of the patient’s need for visual correction. It is also integral to the overall eye health assessment. During our consultation, members provided us with many examples of where a subtle change in refraction in conjunction with clinical experience alerted them to something that ‘didn’t feel right’ and turned out to be asymptomatic disease. Concerns were also raised that separating refraction from the sight test would weaken the ability to detect general health conditions, such as diabetes and hypertension, through characteristic changes to the refractive status.
If we consider a scenario where dispensing opticians were able to perform refraction under the oversight of an optometrist or registered medical practitioner, that would serve to partially mitigate the risk of missed pathology. However, this could produce an unintended consequence in the form of increased pressure on optometrists who are in the role of providing this oversight. Our members, who are GOC registrants, have expressed concern that they may be provided with shorter appointment times that are insufficient to robustly check the refraction. This could increase workplace pressures and lead to clinical errors. These registrants have told us that the clinical governance, audit, and risk measures would need to be sufficiently robust to always ensure patient safety. Clarity of roles and responsibilities was also identified as a key requirement and was felt to be particularly important for locum practitioners.
If the GOC reaches the conclusion that the current statement needs to be withdrawn and replaced with an updated version, it is our view that this should make clear the following points:
- The optometrist, or registered medical practitioner remains responsible for the sight test as defined by the Opticians Act
- An optometrist, or registered medical practitioner may at their discretion be supported by other staff members in the testing of sight, but the decision around what support is provided must remain with the person conducting the sight test
Q16. What would be the advantages, disadvantages and impacts (both positive and negative) of amending or removing our 2013 statement on refraction so that dispensing opticians can refract for the purposes of the sight test? (Impacts can include financial impacts and equality, diversity and inclusion impacts.)
Advantages – none. There is a large and well-trained optometry workforce with sufficient capacity to provide high quality eye care to the population. The limited additional capacity that would be provided by dispensing opticians being able to refract would serve no practical purpose and deliver no obvious benefit to patients.
Disadvantages – the removal of the statement would bring the implied GOC position into open opposition to the Act. The Opticians Act does not refer to ‘refraction’. Indeed, the definition of a sight test given in the Act is what is described as refraction in the GOC’s consultation. The Act mandates requirements additional to refraction to ensure that the appropriate eye health examinations are conducted at the same time as the refraction. In our opinion this fact, in conjunction with the misunderstanding that refraction is not central to the definition of the sight test under the Act, is why the suggestion that refraction could be separated from the sight test is flawed.
As refraction is the central element of the sight test, allowing DOs to refract is a clear breach of the Act and, given that DOs are not able to complete the required additional aspects of the sight test, this would risk both patient safety and the integrity of the sight test.
The removal of the statement on refraction could also be perceived as being more permissive than intended with regard to what is allowed within the confines of the Act. This may have the unintended consequence of encouraging businesses further to erode the public protections enshrined within the Act. Our view is that any move of this kind is unlikely to successfully limit refraction to dispensing opticians only, and moreover may have another unintended consequence of enabling some businesses to start to use other healthcare professionals who may not be adequately trained to provide refraction as part of the sight test. This may introduce unnecessary risk.
Q17. Does the sight testing legislation create any unnecessary regulatory barriers (not including refraction by dispensing opticians)?
AOP response: No
The current legislation does not provide unnecessary regulatory barriers. Instead, it establishes a framework to ensure that when sight is tested, the eye health of the patient is also evaluated. This provides an opportunity for the detection of asymptomatic eye disease that otherwise may not be identified until significant, irreparable damage has already occurred. Given the importance of this role and the potential risk to the public, the profile/type of clinician who is able to conduct sight tests is quite reasonably restricted, to ensure that those testing sight are suitably qualified and trained.
Any changes that seek to break the concurrent delivery of these two aspects of eye care risks an increase in the prevalence of undiagnosed eye disease and late presentation of certain eye conditions. For example, it is recognised that around 50% of glaucoma5 6 is currently undiagnosed. There is a significant risk that patients may not appreciate that a refraction alone will not provide the same assurance as the current sight test. Feedback from our members highlights that many patients confuse various appointments already and anything that further complicates that process could lead to missed pathology. Further there is a long-established tradition of UK patients receiving eye health and refractive elements together. If they are separated, patients may believe that both parts have been completed, increasing the risk that patients do not seek to obtain the eye health component.
We surveyed our members for their views on this element of the GOC’s call for evidence and received 2452 to this question. We asked, “do you agree with the idea that the refraction and health check elements of the sight test could safely be separated?” Nearly 10% of our members agreed that they could, but 87% disagreed, 66% strongly so. In free text responses on our community forums, members pointed out that such a separation presents a real risk of widening the health inequalities gap caused by socio-economic factors (see figure 2 below).
Figure 2
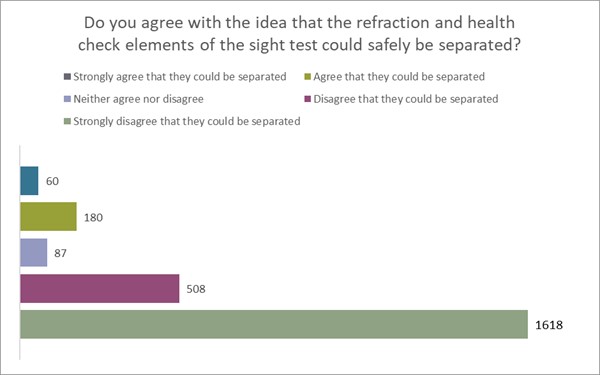
Some other parts of Europe operate a system of split eye health and refraction, or a reduced eye health function. One such example is the Italian optometric system which is predominantly based around refraction with no obligation to detect ocular pathology. A study by Cheloni7 et al reported that in Italy there were several conditions that would likely remain undetected in this type of eye care model. The authors indicated that around 20% of patients may have ocular pathology that required treatment or monitoring and that would be undetected without the requirement for concurrent refraction and eye health examination.
Langis and Forcier8 reviewed data from a large eyecare centre in Canada and retrospectively reviewed records selecting those presenting only with refractive symptoms and then examined whether on further investigation during the eye examination any ocular diagnoses were revealed. They reported that in 26% of patients, asymptomatic disease was found and although the results were numerically different, they were thematically similar.
A larger-scale study of over 6000 eye care records in Canada (Irving et al9) examined the outcome of asymptomatic patients presenting for what they considered to be a routine eye examination and found that around 58% of patients had a change in ocular status or eye care requirements, suggesting that patients are not well placed to judge when there has been a change to the status of their vision or eye health.
If evidence from the literature were extrapolated to the number of sight tests in England it would mean that approximately 11.6m patients would be unlikely to recognise when a change in their prescription requirements or ocular health necessitated re-examination. Based on the work by Cheloni et al, 4 million patients may have pathology that is not monitored assessed or detected if the two components of the sight test are separated. The lost opportunity to detect asymptomatic eye disease could have catastrophic effects on the nation’s eye health. An additional risk is that patients may continue to drive with substandard visual acuity, reduced visual fields, or reduced contrast vision due to the presence of macular degeneration, glaucoma, or cataract.
In an older population it has been shown that outdated, inaccurate, or inappropriate spectacles may contribute to falls10. Also given the high prevalence of cataract in older population groups, and the gradual and often asymptomatic onset of this condition, the separation of eye health from refractive correction could mean a significant number of people living with undiagnosed cataract and consequently an impact upon contrast sensitivity, ability to detect edges (such as stairs) and a consequent increased falls risk. For information, NHS England conducts around 400,000 cataract operations per year, and the vast majority of these will be diagnosed at sight tests.
References
5. Mitchell P, Smith W, Attebo K, et al. Prevalence of open-angle glaucoma in Australia. the blue Mountains eye study. Ophthalmology 1996;103:1661–9
6. Dielemans I, Vingerling JR, Wolfs RC, et al. The prevalence of primary open-angle glaucoma in a population-based study in the Netherlands. The Rotterdam study. Ophthalmology 1994;101:1851–5
7. Cheloni, Riccardo, et al. "Referral in a routine Italian optometric examination: towards an evidence-based model." Scandinavian Journal of Optometry and Visual Science 14.1 (2021): 1-11
8. Michaud, Langis, and Pierre Forcier. "Prevalence of asymptomatic ocular conditions in subjects with refractive-based symptoms." Journal of optometry 7.3 (2014): 153-160
9. Irving, Elizabeth L., et al. "Value of routine eye examinations in asymptomatic patients." Optometry and Vision Science 93.7 (2016): 660-666
10, Lord, Stephen R. "Visual risk factors for falls in older people." Age and ageing 35.suppl_2 (2006): ii42-ii45
Q18. What would be the advantages, disadvantages and impacts (both positive and negative) of sight testing legislation remaining as it is currently? (Impacts can include financial and equality, diversity and inclusion.)
Advantages of retaining current sight testing legislation
Maintaining the link between eye health and the testing of sight (refraction) ensures that patients obtaining refractive correction are provided with reassurance that their eye health is satisfactory. This not only ensures that asymptomatic ocular disease is not present, but patients do not labour under the misconception that they aren’t seeing well simply due to the need for different spectacles, when in fact it is undiagnosed eye disease.
Restricting the testing of sight to the currently defined categories ensures that those who test sight have robust training before being permitted to examine patients.
It ensures that the current English system of nationally funded sight tests for those on means-tested benefits is maintained and that the integrity of the examination is not called into question.
Although much maligned, the national sight test fee ensures that patients have access to affordable eye care. Separation would be likely to increase the cost of the eye health portion as patients would attend less often.
The impact on patients of keeping sight testing legislation as it currently stands is obvious: it helps to protect sight; it reduces the risk of increased avoidable sight loss by ensuring a qualified, appropriately trained clinician is responsible for all elements of the patient’s care. This ultimately helps the NHS to be financially and operationally optimal and efficient. It will also help provide some consistency and continuity to the commissioning and delivery of primary eye care services at both ‘system’ Integrated Care System (ICS) and ‘place’ Integrated Care Board (ICB) level and provide confidence to secondary care ophthalmology in the clinical ability of the profession to take on more enhanced services and procedures.
Disadvantages of retaining current sight testing legislation
We do not recognise any disadvantages to the sight testing legislation remaining as it is, as in our members’ view the current legislation is successful in serving to protect patients. However, we do feel that there could be a small risk that the legislation and/or associated guidance as it stands could restrict innovation and the ability to be flexible and reactive to change at the pace required to keep the profession agile within a complex and fast-paced primary eye care system. In our view this risk is not relevant as technological advancements that are used by the optometrist are already permitted, therefore the Act does not prevent their use.
Q19. Do you have any data on the number/percentage of referrals that are made to secondary care following a sight test / eye examination?
AOP response: Yes
According to historic referral surveys11 around 5% of patients who present for a sight test are referred onwards. Based on the current volume of sight tests conducted in England alone, that equates to around 1m patients who are referred for further investigation in relation to suspect pathological eye conditions.
We have been provided with data by one large group of practices in England who conduct a statistically significant volume of examinations. This data corroborates the historic 5% figure, showing that in just over 6% of cases there was a need for the optometrist to refer the patient or write to the patient’s GP as a result of their examination. Extrapolated to the number of sight tests in England alone, this indicates around 1.2 million patients, which is a slight uplift from the historic estimate that reflects the increased diagnostic capability in optometric practice. Of those patients where referral or information to the GP was required it was for the following reasons:
- Cataract, circa 23%
- Glaucoma, circa 14%
- Anterior eye conditions, circa 10%
- Wet AMD, circa 7%
- Neurological concerns, circa 6%
Again, extrapolating this data for England gives:
- Cataract, circa 280,000 patients
- Glaucoma, circa 170,000 patients
- Anterior eye conditions, circa 120,000 patients
- Wet AMD, circa 84,000 patients
- Neurological concerns, circa 72,000 patients
References
11. Port, M. J. A. "Referrals and notifications by optometrists within the UK: 1988 survey." Ophthalmic and Physiological Optics 9.1 (1989): 31-35
Q20. Are you aware of any data to support or refute the case for separating the refraction from the eye health check?
AOP response: Yes
Soh et al12 conducted a meta-analysis looking at the rate of undiagnosed glaucoma worldwide. They found that adults without annual eye examinations were nine times more likely to have undetected glaucoma and that there was a link between the period between eye examinations and the likelihood for undiagnosed glaucoma to be present. Meanwhile Chua et al13 also found a high prevalence of late glaucoma diagnosis due to the asymptomatic nature of the disease in the early stages.
If refraction and the eye health examination are separated, in our opinion there is an increased risk that patients will not have regular eye health examinations, which could impact the point at which glaucoma is first identified. The associated costs in terms of social care and lost productivity would be significant. RNIB estimate that the indirect costs of associated sight loss in the UK economy are around £6 billion14.
As we have said above, Cheloni et al reported that 20% of patients may have ocular pathology that requires treatment or monitoring and that would be undetected without the requirement for concurrent refraction and eye health examination. If refraction and ocular health examination were to be separated, then around 4m patients would be at risk of eye disease that would previously have been detected.
A robust and detailed comparative analysis of the primary eye care systems in the UK, France and Germany by Thomas et al15 concluded that each system had its own advantages, and all delivered effective services which were capable of high-quality delivery. Whilst this paper does not show that the UK system is unambiguously better than other European countries, it shows that the system already delivers capably and effectively. Thomas et al also concluded that France and Germany should consider increasing the participation of dispensing opticians and optometrists to deal with upcoming challenges, suggesting that the UK has the most sustainable eye care model.
In our opinion, changing the sight test regulatory framework to separate refraction and the eye health check would be a destabilising, risky and costly process, which would jeopardise a system which evidence shows is already working well.
References
12. Soh, Zhi, et al. "The global extent of undetected glaucoma in adults: a systematic review and meta-analysis." Ophthalmology 128.10 (2021): 1393-1404
13. Chua, Jacqueline, et al. "Prevalence, risk factors, and visual features of undiagnosed glaucoma: the Singapore Epidemiology of Eye Diseases Study." JAMA ophthalmology 133.8 (2015): 938-946
14. The economic impact of blindness and sight loss in the UK Adult population RNIB/Deloittes 2013Report (rnib.org.uk)
15. Thomas, D., Weegen, L., Walendzik, A., Wasem, J., & Jahn, R. (2011). Comparative analysis of delivery of primary eye care in three European countries (No. 189). IBES Diskussionsbeitrag
Section 5: Fitting of contact lenses (section 25 of the Act)
Q21. Does the fitting of contact lenses legislation create any unnecessary regulatory barriers?
AOP response: No
To ensure patient safety, it is both clinically appropriate and necessary that contact lens fitting can only be legally carried out by registered optometrists or dispensing opticians with a contact lens specialty. Contact lenses are medical devices which carry numerous risks of harm16 including infection, corneal damage and sight loss. Initial fitting, refits and rechecks with registered optical professionals are vital to ensure that patients are protected from these risks of harm, as we have explained in our answers to questions 22 and in Section 2 and Section 6. The UK has an accessible network of optical practices which are able to offer fittings and follow-on care to patients who want to wear contact lenses.
References
16. Wolffsohn, J. S., Dumbleton, K., Huntjens, B., Kandel, H., Koh, S., Kunnen, C. M., ... & Stapleton, F. (2021). BCLA CLEAR-Evidence-based contact lens practice. Contact Lens and Anterior Eye, 44(2), 368-397
Q22. What would be the advantages, disadvantages and impacts (both positive and negative) of fitting of contact lenses legislation remaining as it is currently? (Impacts can include financial impacts and equality, diversity and inclusion.)
Patients should only initiate contact lens wear under clinical supervision during a fitting with a registered optical professional. GOC-registered practitioners fit contact lenses based on a thorough assessment, taking into account clinical measurements, ocular health and the patient’s individual needs and wellbeing. Once the fitting is complete and patients have been taught how to insert, remove, handle, clean and store the lenses safely, practitioners typically arrange a further check to ensure the lenses are suitable before issuing a specification for supply. The requirement for contact lens fitting to be carried out only by registered optometrists or dispensing opticians helps ensure that patients:
- Understand how safely to wear, store and care for their contact lenses
- Understand how to look for signs of infection or other problems
- Know how to obtain optical care if problems occur
It is encouraging to see that the call for evidence does not suggest that the GOC is considering any changes to fitting regulations. However, in order to ensure that patients are protected from harm and continue to use lenses safely after fitting it is also important that restrictions on sale and supply of contact lenses are kept in place, as we have explained in our answers to Section 6. Taken together this package of regulation helps ensure that patients receive ongoing care from optical professionals as they use their lenses. This includes safety advice about lens use, assurance that lenses are supplied to the correct specification, and management of risks associated with disease and infection. Studies of compliance with advice about contact lens use suggest that regular clinician reviews of care help reinforce patient behaviours that promote safe lens wear.17
References
17. Morgan, P. B., Efron, N., Toshida, H., & Nichols, J. J. (2011). An international analysis of contact lens compliance. Contact Lens and Anterior Eye, 34(5), 223-228
Section 6: Sale and supply of optical appliances (section 27 of the Act)
Q23. Should the sale and supply of optical appliances be further restricted to certain groups of vulnerable patients?
AOP response: Not sure / no opinion
We believe that there is a case to consider adding patients with learning disabilities, older people in residential care and those with complex conditions which increase the likelihood of eye problems to the list of vulnerable groups who need to have optical appliances supplied to them under the supervision of an optometrist, dispensing optician or medical practitioner. It is accepted that vulnerable patient groups may include:
- Those with a dementia disease such as Alzheimer’s
- Adults with a learning disability
- Adults with a complex physical disability
- Older adults in a residential care setting
- Those patients with an existing sight condition such as glaucoma
These groups have a high prevalence of eye disease18, 19 which means that they would benefit from having their optical appliances provided under clinical supervision.
The case for adding these groups would also be strengthened if the GOC is considering the removal of restrictions on the sale and supply of optical appliances which apply to all patients.
However, this should only be done if this has support from patient organisations who represent these vulnerable groups. Practical issues of implementing these restrictions and whether the changes will lead to overall benefit and protections for patients are important considerations.
References
19. Emerson E, Robertson J The estimated prevalence of visual impairment among people with learning disabilities in the UK (2011)
Q24. If you answered yes to the previous question, what would be the advantages, disadvantages and impacts (both positive and negative) of further restricting the sale and supply of optical appliances to certain groups of vulnerable patients? (Impacts can include financial and equality, diversity and inclusion.)
The higher prevalence of eye disease in these groups means that many patients will have eye conditions for which it is beneficial to have clinical supervision alongside the supply of optical appliances as well as additional interventions where appropriate:
- A specialist lens or fitting for the optical appliances may be needed
- Contact with a practitioner could help identify changes to the patient's eye condition or corrective needs
- The patient could be referred to another service or given advice to support them with their current clinical, support or visual corrective needs
- The risk of falls in older people could be mitigated by appropriate visual correction intervention
The main disadvantages of adding these groups to the list of vulnerable groups is that many people within these vulnerable groups may not require additional support. This could lead to issues and perceptions of unfairness and barriers to access for them. It may also create an increased cost burden on these groups. Provision of clinical support for people with learning disabilities is likely to work better in areas where targeted eye care and support services have been commissioned, but this varies across the UK.
There would also be practical challenges in how to identify the patients who belong to these vulnerable groups. Registers for people with learning disabilities, living with complex conditions and in residential care do exist, but there is no national register equivalent to that for people with a visual impairment.
Q25. Do the general direction / supervision legislative requirements relating to the sale of prescription contact lenses create any unnecessary regulatory barriers?
AOP response: No
We do not think that the current legislation creates unnecessary regulatory barriers. The current restrictions are in place to ensure that patients have a safe level of clinical assurance that they are being supplied with appropriate lenses and that the patient has had a recent sight test and contact lens fitting. We have explained the risks of these restrictions being relaxed or removed in our answer to question 26, and throughout this section.
Q26. Would there be a risk of harm to patients if the general direction / supervision requirements relating to the sale of prescription contact lenses changed?
AOP response: Yes
The current process provides safeguards to protect patients by ensuring that:
- The lenses being supplied match the specification, and the choice of lens is in the patient’s best interest
- That the specification is accurate and based on a recent contact lens fitting and sight test
Removal or relaxation of these supply restrictions could lead to a risk of patients using contact lenses without regular refits and rechecks with an optical professional, or in some cases having never being fitted for them. Contact lens wear carries various risks of infection and corneal damage.20 As we explained in our answer to Section 5 and throughout this section, without appropriate advice about how to care for, clean, and store their lenses, there is a significant risk that patients develop unsafe contact lens habits. This may increase the risk of developing sight loss-causing infections.21
Acanthamoeba keratitis is a rare but severe corneal infection that affects mainly contact lens wearers in Western countries.22 It can lead to visual impairment and blindness, and it can develop as a result of poor contact lens hygiene and exposure to tap water. This is why regulations that ensure the patient has had a recent contact lens fitting and provision of aftercare are essential. Our answers to the questions about zero-powered lenses further explain why non-legislative approaches are insufficient to ensure public protection.
There are also several risks of harm to patients from being supplied with lenses that do not match their specification, which can result from a failure to verify contact lens particulars or inappropriate contact lens substitution. The problems that can result are set out in our answer to question 28 and these include risks of corneal damage and eye infection, poorer visual fit, acuity and eye discomfort, and a risk of failing to meet vision standards for driving.
Proportionate modification of the requirement
We disagree with the GOC’s reasoning about the contact lenses verification requirement being ‘outdated’, but there may be ways sensibly to update requirements whilst also maintaining safeguards to ensure patients are being appropriately and safely supplied. Current legislation requires that the seller should verify the specification with the prescriber when supplying under general direction (unless the patient is able to supply an original in-date specification).
We think it would be acceptable to relax this requirement by:
- Allowing electronic copies (such as a photo, or scan) of the specification to be provided without the need for verification
- Requiring the seller to verify the specification with the original prescriber if there is any doubt about what the particulars of the specification are, for example if they cannot be read properly or if there is anything to suggest that it may have been tampered with
References
20. Wolffsohn, J. S., Dumbleton, K., Huntjens, B., Kandel, H., Koh, S., Kunnen, C. M., ... & Stapleton, F. (2021). BCLA CLEAR-Evidence-based contact lens practice. Contact Lens and Anterior Eye, 44(2), 368-397
21. Stapleton, F., Keay, L., Jalbert, I., & Cole, N. (2007). The epidemiology of contact lens related infiltrates. Optometry and vision science, 84(4), 257-272
22. Carnt, N., & Stapleton, F. (2016). Strategies for the prevention of contact lens‐related Acanthamoeba keratitis: a review. Ophthalmic and physiological optics, 36(2), 77-92
Q27. Do the legislative requirements for verification of contact lens specifications create any unnecessary regulatory barriers?
AOP response: No
The requirement to verify a contact lens specification when supply is under general direction, and the original has not been provided, is a safeguard to ensure that patients are supplied with the appropriate lenses without a risk of detrimental impacts. The risks that can result from being supplied with lenses that do not match the specification include corneal damage and eye infection, poorer visual fit, acuity and eye strain, and a risk of failing to meet vision standards for driving. We have provided more detail about these harms in our response to question 28. Verification of the specification also provides assurance that the patient has had a recent contact lens fitting, and better enables the seller to make reasonable aftercare arrangements to protect the patient from risks of harm related to contact lens wear.
A key problem with the current regulatory situation is that sellers implement the existing legislative requirement to verify a contact lens specification in a variety of ways, and some sellers, especially those based online, fail to fulfil the current public protection requirements.
The need to verify the specification should remain in place where there is doubt about the particulars of the specification, although there is scope safely to update the verification requirement to allow acceptance of electronic specifications (as we have set out in our answer to question 28). It is important that any changes to the current requirements are properly enforced by the GOC, and breaches are dealt with through regulatory action based on its illegal practice protocol to ensure consistent application of verification requirements by sellers. We have proposed ways that the GOC can deal with online sellers breaching the Opticians Act, including those based overseas, in our answers to questions 50 and 51 in Section 7.
Q28. What would be the advantages, disadvantages and impacts (both positive and negative) of removing the requirement to verify a copy of or the particulars of a contact lens specification? (Impacts can include financial and equality, diversity and inclusion.)
Risks of inappropriate lenses being supplied
The reason this requirement is in place is to ensure that the lenses being supplied by the seller correctly match the patient’s contact lens specification. Removing the requirement to verify the specification when an original has not been provided will increase the probability that patients are supplied with inappropriate lenses not matching their specification. Detrimental consequences for patients can result both from being supplied with an incorrect lens power, but more seriously from poorly fitting lenses that do not match an individual’s eye shape. Resulting patient harms include:
- Lenses that cause mechanical damage to the cornea due to a poor fit, or lead to corneal damage as a result of physical characteristics of the lens, such as how well the surface stays hydrated
- The oxygen transmission of the lens may not be as anticipated by the prescribing practitioner when providing advice on how long it is safe to wear the lenses
- Corneal damage can increase the risk of eye infections including potential sight-threatening diseases such as microbial keratitis
- Contact lenses that fit poorly can reduce visual acuity and lead to discomfort. This is a particular concern with varifocal contact lenses which have more complex prescriptions
- Where a patient only just meets the required visual acuity standard for driving when vision is corrected by contact lenses, a contact lens which does not match their current specification could lead to the patient not meeting the required standard, potentially leading to them driving illegally and risking harm to the patient and other road users
Risks of reduced contact with optical professionals
Removal of the requirement to verify the contact lens specification will mean that patients may be able to purchase lenses simply by providing their spectacle prescription to the seller or, worse, by simply guessing. This would increase the risk that patients delay attending for a contact lens fitting beyond two years. The risk of this happening has increased due to the increased availability of contact lenses online, and a recent study of patients buying contact lenses on the internet found a growing number who initiated lens wear independently without professional care and advice.23 Less frequent contact lens care and advice from optical professionals will increase the risk of patients developing poor contact lens hygiene habits and increasing the risk of developing harmful infections that could lead to sight loss, as we have explained in our answer to question 26. If patients reduce their engagement with optical professionals, there will also be reduced opportunities for emerging risks and signs of infection and corneal damage to be identified.
Problems with drawing lessons from the COVID-19 pandemic
In its call for evidence the GOC says that during the COVID-19 pandemic it relaxed the verification requirement ‘without any detrimental effects that it knows of’. The problem with using this as a basis for changing the verification requirement is that:
- It is still too early to evaluate the impacts of relaxing regulatory restrictions during the pandemic and problems that have resulted from this may still come to light, potential sight loss from delays in diagnosis of eye disease take time to develop
- Contact lens use went down during the pandemic as people were in lockdown24, which may have led to reduced prevalence of problems related to contact lenses
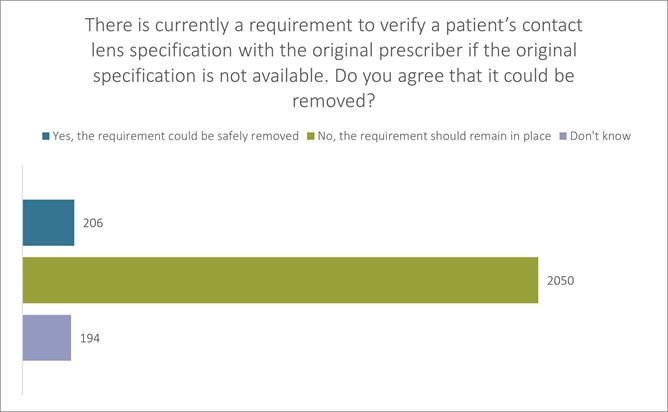
We consulted our members on this question, receiving 2450 responses. We asked, “there is currently a requirement to verify a patient’s contact lens specification with the original prescriber if the original specification is not available (such as when supplying contact lenses online). The GOC is consulting over whether this requirement could safely be removed. Do you agree that it could be removed?”
8% agreed that the requirement could be safely removed, but 84% said that it should remain in place (The remaining 8% said they did not have a view). (see figure 3 below).
Figure 3
Furthermore, we also consulted our members on the following question, receiving 2450 responses. We asked, “Does the GOC requirement to have a sight test within the last 2 years, before contact lenses are fitted/refitted, help to protect patients?” 94% agreed that the current GOC requirement does help protect patients (see figure 4 below).
Figure 4
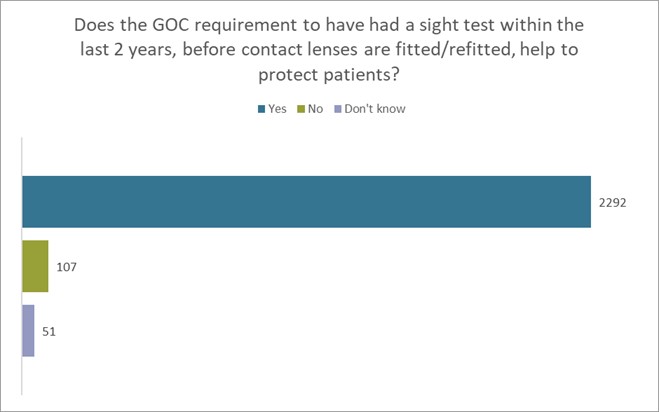
Proportionate modification of the requirement
We disagree with the GOC’s reasoning about the contact lenses verification requirement being ‘outdated’, but there may be ways sensibly to update requirements while also maintaining safeguards to ensure patients are being supplied appropriately and safely. Current legislation requires that the seller should verify the specification with the prescriber when supplying under general direction, unless the patient is able to supply an original in-date specification. We think it would be acceptable to relax this requirement by:
- Allowing electronic copies (such as a photo, or scan) of the specification to be provided without the need for verification
- Requiring the seller to verify the specification with the original prescriber if there is any doubt about what the particulars of the specification are, for example if they cannot be read properly or if there is anything to suggest that it may have been tampered with
References
23. Mingo-Botín, D., Zamora, J., Arnalich-Montiel, F., & Muñoz-Negrete, F. J. (2020). Characteristics, behaviors, and awareness of contact lens wearers purchasing lenses over the internet. Eye & Contact Lens, 46(4), 208-213
24. Morgan, P. B. (2020). Contact lens wear during the COVID-19 pandemic. Contact Lens and Anterior Eye, 43(3), 213
Q29. Do you think the Act should specify a definition of aftercare? (If yes please specify what you think the definition of aftercare should be.)
AOP response: Yes
Current situation
Because aftercare is not defined in the Act, optical professionals meet this requirement in different ways – as follow-on care which helps protect patients from the risks of harm associated with contact lens use. Many practitioners refer to a recheck appointment or refitting and reissuing of a contact lens specification at its expiry as being part of aftercare. While this may be true, in our opinion it does not address the aftercare requirement as mandated by the Act. Professional bodies for optometrists provide the following advice about delivering the aftercare requirement:
- Our own advice to AOP members advises that they “give instructions on the use and care of the lenses”, and “Use your clinical judgement to decide when to call your patient back for a contact lens check-up or eye examination”
- College of Optometrists professional guidance on aftercare advises practitioners to make aftercare provision “as far as and for as long as may be reasonable” and that it should include “practical arrangements for aftercare with an appropriate provider”. It goes on to list a range of provisions including: a point of contact for the patient; advice about lens use and patient advice on identifying risk of infection; a contact lens helpline; monitoring aftercare arrangements
- The General Optical Council (Contact Lens (Qualifications etc.) Rules) Order of Council 1988, refers to aftercare and states, “The fitting of contact lenses shall be accompanied by the necessary instruction and information to the person fitted on the care, wearing, treatment, cleaning and maintenance of such lenses and the person fitting such lenses shall be obliged to provide for clinical management and adjustment of the fitting thereof for a period of six months from the date of first fitting.” Modern single use lenses rarely need these adjustments. However, it was clearly the intention that the person fitting lenses should provide aftercare.
Defining aftercare for contact lens suppliers
In the past, the vast majority of patients purchased their supply of contact lenses from the same provider that carried out the contact lens fitting or refit and, in that scenario, the 1988 reference to aftercare would have previously been sufficient to ensure patients received appropriate follow-on care. However, as the online market for contact lenses grows, there has been and is likely to be an increasing separation of fitting from supply. This makes the sellers’ duty of aftercare even more important to protect patients. Providing a definition of aftercare could help to ensure that patients who purchase from online suppliers are given suitable advice on how to safely use their lenses and what to do if they experience problems with them. Our suggested definition for aftercare would be:
‘To ensure that those who sell or supply contact lenses to patients are mandated to ensure follow-on arrangements for care are in place, which provides the patient with a reasonable means to safely use the supplied contact lenses, identify signs of infection or other harm and to obtain suitable care or advice if problems occur. It would seem reasonable to draw inspiration from the 1988 rules, but with the onus on the supplier’.
It is vital that any new definition of aftercare is consulted on by the GOC in order to avoid unintended consequences, ensure that it can be reasonably implemented by suppliers and that it can be consistently applied as a duty on all sellers, including those based solely online.
Q30. Does the zero powered contact lenses legislation create any unnecessary regulatory barriers?
AOP response: No
The requirement for zero-powered lenses to be sold by or under the supervision of a registered professional is necessary and proportionate in order to protect the public from serious risk of harm through eye infection which could lead to sight loss. The risk to eye health from contact lenses is not defined by the power of the lenses, but the way it is worn, handled, and cared for; as such we see no reason that zero-powered lenses should be treated more leniently than power lenses. The UK has a large and accessible network of primary care optometric practices which can fit and supply the public with cosmetic lenses and provide them with advice about safe lens use and storage, and aftercare in order to protect them from these harms.
Q31. Would there be a risk of harm to patients if the requirements relating to the sale of zero powered contact lenses change?
AOP response: Yes
The physiological risk of serious harm resulting from eye infection, including sight loss, can result from the unsafe use and storage of cosmetic lenses in the same way as from prescription lenses for vision correction.25 Without appropriate advice about how to care for, clean and store their lenses there is a significant risk that lets users develop poor habits that could lead to sight-threatening infections. Acanthamoeba keratitis is a rare but devastating corneal infection that affects mainly contact lens wearers in Western countries.26 It can lead to visual impairment and blindness; it can develop as a result of poor contact lens hygiene and exposure of the contact lenses to tap water.
Other forms of microbial keratitis are also associated with poor lens hygiene and can lead serious harmful eye infections, but acanthamoeba carries the highest risk of harmful consequences.27
References
25. Kerr, N. M., & Ormonde, S. (2008). Acanthamoeba keratitis associated with cosmetic contact lens wear. The New Zealand Medical Journal (Online), 121(1286)
26. Carnt, N., & Stapleton, F. (2016). Strategies for the prevention of contact lens‐related Acanthamoeba keratitis: a review. Ophthalmic and physiological optics, 36(2), 77-92
27. Carnt, N., Samarawickrama, C., White, A., & Stapleton, F. (2017). The diagnosis and management of contact lens‐related microbial keratitis. Clinical and Experimental Optometry, 100(5), 482-493
Q32. If you answered yes to the previous question, is legislation necessary to mitigate this risk?
AOP response: Yes
We believe that the current legislation protects users of zero-powered lenses and is necessary. There would be a significant risk of increased harm to wearers of cosmetic lenses if current restrictions on the sale of zero-powered lenses are relaxed or removed because:
- The removal or relaxation of regulation on the sale of zero-powered lenses could send out the incorrect and dangerous message to the public that there aren’t any risks associated with the use of cosmetic lenses
- A lack of regulation will increase the likelihood of individuals purchasing their lenses without any advice about wearing and caring for their lenses from a professional registrant. Tellingly, a multi-centre study28 in France found that the likelihood of microbial keratitis infection developing in lens wearers was increased by a factor of 12.3 when an eye care profession was not involved in the sale of cosmetic lenses
- New sellers are likely to start marketing and selling cosmetic lenses to users if current sales restrictions are removed:
- Sellers may fail to provide sufficient advice about lens care to protect patients
- Even if sellers understand the risks, they may underplay these to customers, as they wouldn’t need to meet the same standards of care as professional registrants
- Prevalence of harmful eye infection associated with cosmetic lens use is highest amongst young people29, and this group will be harder to reach with advice about safe lens use
- The most significant harm would be to individual lens wearers who suffer sight loss as a result of infection, but an increasing number of infections could also lead to increased pressures for already overstretched hospital eye services and further delays for all patients
Non-legislative approaches to public protection
While legislative restrictions should remain in place for the sale of zero-powered lenses, other approaches should also continue to be used to ensure that cosmetic lens users are protected from harm. Legislative restrictions reduce the risks of harm, but sellers are likely to continue to operate outside the legal framework and zero powered lens users will need additional messages about how to reduce their risks of harm from lens wear.
Public education about the risks of cosmetic lenses is important and can help reduce the risk of harm. The AOP has regularly produced advice and information for patients and professionals about the risks of wearing cosmetic lenses, such as this press release about novelty lenses at Halloween. However, the younger age group who are the most likely to engage in more risky lens use behaviours which lead to harmful infection are also one of the hardest demographics to reach with public health advice. Safe lens use campaigns such as Love your Lenses are helpful at reducing harm and have rightly received support from the GOC, AOP and other bodies. However, these safety messages are most likely to work at reinforcing safety advice for existing lens users who have engaged with eye care services. Some cosmetic lens users may have never previously had a contact lens fitting, or even a sight test or contact with any eye care service.
Contact lens packaging could also carry prominent warnings about the risks of poor contact lens hygiene and usage. This approach has been used effectively in differing ways for cigarette and alcohol packaging. However, this would itself require legislation. It is unlikely that manufacturers would voluntarily add such warnings without being mandated or incentivised to do so.
References
28. 937–944. 14. Sauer A, Bourcier T, French Study Group for Contact Lenses Related Microbial K. Microbial keratitis as a foreseeable complication of cosmetic contact lenses: a prospective study. Acta Ophthalmol 2011; 89: e439–442
29. Gray, T. B., Cursons, R. T., Sherwan, J. F., & Rose, P. R. (1995). Acanthamoeba, bacterial, and fungal contamination of contact lens storage cases. British Journal of Ophthalmology, 79(6), 601-605
Q33. What would be the advantages, disadvantages and impacts (both positive and negative) of zero powered contact lenses legislation remaining as it is currently? (Impacts can include financial and equality, diversity and inclusion.)
We believe it is important for public protection that the legislation around zero-powered lenses remains. The advantages of this are:
- The legal requirement about the sale and supply of zero-powered lenses sends out a clear message that they are a regulated medical device and need to be used with appropriate clinical advice and care in the same way as prescription lenses to avoid risk of harm
- Evidence shows that cosmetic lens users are less likely to suffer infections that can lead to severe sight loss if they have appropriate advice from an eye care professional on purchase
- The current restrictions protect young people, as the demographic who are the most likely to be users of cosmetic lenses
The potential disadvantages of retaining the current restrictions centre around challenges in enforcing the current regulations and dealing with the risks associated with online sales:
- The GOC may lack appropriate resources of enforcements to tackle all UK websites and retail outlets selling cosmetic lenses without oversight from an optical professional
- The GOC may find it particularly challenging to mitigate the risks to the public from non-UK sales of cosmetic lenses, as consumers increasingly switch to online purchasing habits – again there is likely to be a disproportionate risk of harm to young people who have not had a contact lens fitting
These disadvantages and resultant eye health risks can be mitigated by the GOC taking a strong public protection approach to its enforcement activity based on its illegal practice protocol. This includes taking action against illegal sales in UK retail outlets (such as fancy-dress shops) and referral to trading standards officers where necessary. The GOC should also develop a better strategy to deal with patient safety risks arising from online sales and from sellers based outside the UK as we have explained in our answers to question 50 and 51. The GOC should consider whether it needs new powers in order to properly achieve this.
Q34. Are there any unnecessary regulatory barriers in the Act that would prevent current or future development in the sale of optical appliances or competition in the market?
AOP response: No
Q35. If you answered yes to the previous question, what would be the risk on the consumer if these barriers were removed?
AOP response: N/A
Q36. Is legislation regarding the sale of optical appliances necessary to protect consumers (except restricted categories)?
AOP response: Yes
It is clear from the substantial evidence of risk30 of harm to patients that can result from sale and supply regulations not being followed that continued GOC enforcement of this legislation is necessary to protect the public. The current restrictions fundamentally help protect the public from two key areas of risk:
- Risks of developing severe eye infection that can lead to sight loss, associated with a failure to engage with eye care professional advice and safely care for contact lenses
- Risks of developing eye disease that is asymptomatic and can lead to sight loss, associated with purchasing optical appliances for vision correction without attending a sight test which can detect the early sign of pathology
The whole package of current sale and supply restrictions works to protect patients from these areas of risks which can lead to severe detrimental consequences of sight loss and disease. As we explain across our answers to this section, there are a small minority of areas which may require some revision, but otherwise legislative restrictions on sale and supply should remain in place and be enforced by the GOC as part of the illegal practice strategy. In fact, the Europe Economics risks assessment, commissioned by the GOC in 201431 to identify the costs of illegal practice, concluded that “misdiagnosis of diseases has both a high severity of an adverse event, and a medium-high likelihood of an adverse event occurring under illegal practice”.
GOC enforcement
Given the risks of harm related to optical appliances, we are concerned by the GOC’s view, expressed in the call for evidence, that it is not practicable for it to pursue prosecutions in the magistrates’ court for breaches of UK law relating to legal restrictions on sale and supply of optical appliances. Prosecution is rightly the final step of enforcement but is necessary when other attempts at encouraging regulatory compliance fail, in order to challenge harmful practice by UK sellers which can lead to patient harm.
We accept that the GOC faces challenges when it comes to encouraging online sellers based overseas to comply with UK legislation. However, the GOC’s recent revisions to its illegal practice protocol include strategies to help address this – such as contacting the online marketplace where optical appliances sales are listed. In order to protect the public from harm the GOC needs to strengthen this approach in the ways we have set in our answer to questions 50 and 51. This includes improving public education about risks, contacting the seller and developing a way that patients can identify which sellers are operating within UK law.
References
30. The evidence of harms is referenced throughout our answers to this section on sale and supply restrictions
31. Europe Economics report: health risk assessment for illegal optical practice (2014), GOC website
Q37. Is the two-year prescription restriction on purchase of spectacles from non-registrants an unnecessary regulatory barrier?
AOP response: No
It is vital that this requirement is kept in place in order to protect patients from the risk of severe harm and sight loss. As we have explained in our answer to question 38, removing it would increase the risk of delayed diagnosis of severe eye disease and other detrimental impacts.
Q38. What would be advantages, disadvantages and impacts (both positive and negative) of patients being able to purchase spectacles from non-registrants without a prescription dated in the previous two years? (Impacts can include financial and equality, diversity and inclusion.)
AOP response: No
The removal of this requirement would increase the likelihood that patients delay getting a sight test beyond the recommended two years, increasing the risks of delayed diagnosis of severe eye disease and using spectacles which are unsuitable for their current vision correction needs. In fact, the risk that patients delay or forgo sight tests have increased over time due to the emerging availability of unregulated online self-refraction services. The risks associated with removing the requirement for an in-date spectacle prescription are likely to exacerbate health inequalities as patients who are less health literate will be most likely to delay sight tests, and severe disease such as glaucoma disproportionately impacts some ethnic groups more than others. It could also delay the detection of systemic conditions through case findings during the sight test, such as cardiovascular disease in groups who are otherwise unlikely to engage with healthcare professionals.
The GOC call for evidence says that ‘some patients are not happy with this requirement [for an in-date prescription]’. However, this is a very weak basis for considering change to regulations that would introduce risks of harm for the whole patient population. It may also suggest that patients are not always aware about the important role the sight test plays in safeguarding their eye heath.
Removing the current requirement for an in-date prescription for spectacle sales could also increase the exposure of patients to mis-selling by non-registrants.
Delayed diagnosis of eye disease
Late diagnosis of eye conditions can lead to irreversible sight loss, damaging patients’ quality of life and imposing significant wider costs on society.32 This is a specific risk for glaucoma, which is asymptomatic in its early stages, particularly when it only affects one eye. In the UK, diagnosis of glaucoma typically occurs opportunistically during routine sight tests in primary care optometry, and patients who do not attend sight tests are most at risk of only presenting when they have advanced stage disease when permanent harm has already occurred.33 Early diagnosis can prevent the disease causing permanent and severe damage to vision and quality of life.
However, opportunities for early diagnosis will be reduced if patients do not attend for sight tests following a removal of the requirement to have an in-date prescription for spectacles. In the absence of population or risk-based screening for glaucoma34, ensuring patients attend regular sight tests is the best approach to early identification of the disease. Detrimental impacts from missed diagnosis could be significant, as glaucoma has a prevalence of about 1% in the UK, but closer to 2% for over 40s and 5% for those over 75. This could disproportionately impact Black and Asian patients who have a higher risk of developing different forms of glaucoma compared to the White population.35 Black patients have nearly a three times greater risk of developing open angle glaucoma.36 The overall disease burden will also increase in the future due to demographic trends as the UK population ages.37
Table 1 Risk factors associated with primary open-angle glaucoma Estimates from key meta-analyses (95% CI). Reproduced from SIGN 144
| Age | Prevalence (%) | |
| 80 | 7.8 (5.2-12) | |
| 70-79 | 5.1 (3.6-7.2) | |
| 60-69 | 3.7 (2.7-5.0) | |
| 50-59 | 2.2 (1.6-3.0) | |
| 40-49 | 1.3 (0.9-1.9) | |
| 30-39 | 1.6 (0.7-3.8) | |
| Black ethnicity | Age-adjusted prevalence % | Odds ratio |
| 7.5 (6.8-8.4) | 2.9 (1.4-5.9) |
Opportunities to detect early signs of age-related macular degeneration (AMD) and retinopathy are also likely to be reduced, but the impacts would be less severe as these diseases have early-stage symptoms, however the delay could lead to missed opportunities for lifestyle-based interventions to reduce risk of severe disease.
Spectacles that do not meet current vision correction needs
Patients delaying attendance at a sight test may not realise that their vision correction needs have changed. The removal of the current restriction could lead to more patients wearing spectacles which are not well-suited to their current correction needs. This would lead to the following risks:
- Where a patient only just meets the required visual acuity standard for driving when vision is corrected by spectacles, purchasing spectacles which do not match their current vision correction needs could lead to the patient not meeting the required standard – leading to a risk of harm to the patient and the public
- Increased risks of visual discomfort, headaches and potentially effects on binocular vision, plus an increased falls risk for older patients
References
32. Rouland, J. F., Berdeaux, G., & Lafuma, A. (2005). The economic burden of glaucoma and ocular hypertension. Drugs & aging, 22(4), 315-321
33. Kastner, A., & King, A. J. (2020). Advanced glaucoma at diagnosis: current perspectives. Eye, 34(1), 116-128
34. Hamid, S., Desai, P., Hysi, P., Burr, J. M., & Khawaja, A. P. (2022). Population screening for glaucoma in UK: current recommendations and future directions. Eye, 36(3), 504-509
35. Glaucoma information, Moorfield’s Eye Hospital (website accessed June 2022)
36. SIGN (2015) Glaucoma referral and safe discharge (SIGN 144). Scottish Intercollegiate Guidelines Network. www.sign.ac.uk
37. Day, F., Buchan, J. C., Cassells-Brown, A., Fear, J., Dixon, R., & Wood, F. (2010). A glaucoma equity profile: correlating disease distribution with service provision and uptake in a population in Northern England, UK. Eye, 24(9), 1478-1485
Q39. What would be advantages, disadvantages and impacts (both positive and negative) of the legislation remaining as it is currently? (Impacts can include financial and equality, diversity and inclusion.)
The requirement for non-registrants to sell or supply spectacles only in accordance with a prescription issued in the last two years provides a strong level of public protection. The main benefit is that it increases the likelihood that patients attend regularly for sight tests with an optometrist. This creates an opportunity for:
- Detection of the early signs of severe eye disease, especially asymptomatic conditions such as glaucoma, through the eye health portion of the sight test
- Patients to be supplied with prescription spectacles based on a recent prescription which is likely to be a more accurate reflection of their current visual requirements
- A message to be given to patients that a sight test is recommended every two years
Early detection of the signs of a range of eye disease is enabled by regular sight tests. As we have explained in our answer to question 38, this is particularly important for early diagnosis of glaucoma, for which there is no screening programme, and which is symptomless at initial onset and a condition where any damage that has occurred cannot be reversed.
Accessibility of sight tests and consumer choice
The GOC call for evidence says that some patient groups believe this restriction limits consumer choice. We don’t believe this is a strong argument because:
- Patients across the UK can easily arrange sight test appointments through accessible optical practices located in high streets, supermarkets, health centres and other locations. There are also a wide range and choice of providers
- Waiting times to book a sight test are minimal across most practices
- The sight test is competitively priced due to the level of competition within the market, and around 70% of patients receive an NHS-funded sight test. This includes children, over-60s, those with diabetes, with or at risk of glaucoma, the visually impaired and those on means tested benefits. And, in Scotland eligibility for NHS sight tests is universal
- The Act already contains a protection that means there is no obligation to purchase spectacles from the practice that delivered the sight test
- The Act also ensures that patients are provided with a copy of their prescription once the sight test is completed. This allows patients to choose where and from whom they purchase spectacles
Q40. Does the legislation in relation to the sale and supply of sportswear optical appliances for children under 16 create any unnecessary regulatory barriers?
AOP response: No
We have asked our members and have no evidence, anecdotal or otherwise to suggest this is is an issue for paediatric patients or their families. We feel that robust legislation and associated guidance is essential to ensuring good eye health and care in children.
Q41. What would be advantages, disadvantages and impacts (both positive and negative) of children under 16 being able to buy sportwear optical appliances outside the supervision of a registrant / registered medical practitioner? (Impacts can include financial and equality, diversity and inclusion.)
Unsupervised sales of optical appliances to children are a significant area of risk. This is because children’s eyes are still developing, and poorly fitting and inappropriate prescription eyewear, provided without appropriate clinical oversight, can lead to discomfort.38 There is also a risk that the incorrect impact protection may be provided if the process is not overseen by registrants. The call for evidence says that risks associated with sports eyewear may be less because the appliances are being worn infrequently. However, the problem of children having inappropriate vision correction leading to harm are made more significant because they will be engaging in higher risk activities such as swimming, sports, and outdoor pursuits.
References
38. Powell C, Wedner S, Hatt SR (2009) ‘Vision screening for correctable visual acuity deficits in school-age children and adolescents’ The Cochrane Collaboration
Q42. What would be advantages, disadvantages and impacts (both positive and negative) of the legislation remaining as it is currently? (Impacts can include financial and equality, diversity and inclusion.)
See answer to question 41.
Q.43 Are there any other aspects of the sale and supply of optical appliances legislation that you think need changing or create unnecessary regulatory barriers?
AOP response: No
Q44. What would be the advantages, disadvantages and impacts (both positive and negative) of the sale and supply of optical appliances legislation remaining as it is currently? (Impacts can include financial and equality, diversity and inclusion).
We feel the current legislation works well as it protects patients and the public from harm. As previously stated above, the legislation should remain in order to continue to maintain a risk-based approach. This will also help in enabling ICSs to better commission and deliver consistent and equitable care.
Section 7: Delivery of remote care and technology
Q45. Do you have any knowledge or experience of areas of technological development that the GOC should be aware of when considering changes to the Act?
AOP response: Yes
AI, technology and remote care are different things and require separate consideration, although we note that at times they are used interchangeably.
AI is often referred to as the science and engineering of making intelligent machines, especially intelligent computer programs. AI takes different forms; narrow or ‘weak’ AI is task-specific, such as virtual assistants Siri and Alexa. Strong AI is a more theoretical concept where the AI would have human-like intelligence. Machine learning and deep learning are subsets of AI, with deep learning, being a subset of machine learning.
Technology is a broad term and can mean many things. It could encompass AI but should not be used interchangeably. One dictionary definition of technology is, “Technology refers to methods, systems, and devices which are the result of scientific knowledge being used for practical purposes.”
This definition could be applied to practically every piece of equipment that has ever been used to conduct a sight test, or eye examination. As such it is important to avoid the mistake of presuming that technology describes something new and so far, unseen.
Remote care is also a broad term. It can be used to describe the interaction of a patient with a healthcare professional via telephone or videocall, but it can also provide a description of the interaction between two clinicians to collaborate on or share the care of a patient.
In our answers to the questions in this section we attempt to make the distinction and draw out the different considerations.
We welcome the benefits that technology has already brought to the sector and agree that the potential benefits of future developments in AI and technology may be significant.
Optical coherence tomography (OCT) is now a well-established technology and has been a daily benefit to patients in practice. It is having a substantial impact on outcomes, by allowing better diagnosis, management and monitoring and has enhanced patient understanding of the clinical importance of sight testing.
OCT provides a detailed view of the inner parts of the retina, and this has helped to reduce referrals where there is not a known cause for reduced vision by ruling out some sinister causes. In cases like central serous retinopathy this allows for a definitive diagnosis and avoids confusion with similar but more serious conditions such as Wet AMD.
Technological developments in AI, including machine learning and deep learning, could mean that the large, widely distributed optometric workforce will be able to play a much greater role in delivering eye care and reducing pressure on the hospital eye service.
To achieve that goal, policymakers will need to lay the foundations by:
- Building the understanding of aspects of AI into optometrists’ training and professional development
- Driving the design and commissioning of eye healthcare services in which primary optometrists routinely use AI to make quick and accurate diagnoses and referrals
- Ensuring that the databases and algorithms that underpin AI are suitably overseen and free from bias
We have seen the huge benefits in the sector and for the patient in the optometrist-led remote care which was delivered during the COVID pandemic. Remote care was an advantageous tool especially for patients who couldn’t attend a face-to-face appointment for health risks or who were unable to travel. While the delivery of remote care existed pre-COVID, the sector witnessed an acceleration in the provision of remote care during the early part of the pandemic, especially in the primary care sector with services such as Community Urgent Eyecare Services (CUES) and Minor Eye Conditions Service (MECS). The delivery of remote care in the secondary sector is more established with remote hubs being an integral part of hospital eye care services. As we have outlined above, it is important to note that remote care has several variants, and it is important to be clear which variant is being discussed.
One use of remote care is in follow-up examinations of chronic conditions which have already been treated, or where an established management plan is in place. Glaucoma, for example, can be managed with remote collaboration between primary and secondary care clinicians after the relevant clinical information (e.g., OCT; IOPs; visual fields) has been obtained. This may mean that more patients could be treated in primary care which will help to reduce pressure on the secondary care sector.
There is an opportunity to increase the drive to design and commission eye healthcare services where primary care optometrists routinely utilise AI to increase the speed and accuracy of their diagnosis. If implemented properly, it could allow primary care to deliver secondary care level expertise to patients in a primary care setting.
A priority is to ensure that healthcare transformation and commissioning takes place that enables better utilisation of the primary care optical sector to ease significant workload pressures affecting hospital eye services and the additional backlog of unmet patient eye care needs created by the pandemic (for example through the commissioning of pre- and post-operative cataract services and management, or co-management of glaucoma)
There is a need for investment within the sector so that we can realise the potential new technology can bring. Initial costs of hardware and software are a barrier for some businesses. This creates a two-tier service and exacerbates regional health inequalities. In some of the lowest socioeconomic settings, patients may not be able to access OCT and similar technology as the cost of the additional examination is currently unfunded.
Q46. Is there any evidence that increased use of technology or remote care may have an impact on patient safety or care in the future?
AOP response: Not Sure
We feel that the construction of this question doesn’t take into account the use of technology and remote care currently in optometry nor the opportunities for further technological innovation in this field. There are without doubt significant benefits to patients in the use of technology and remote care across all areas of clinical practice, which is well documented and has demonstrated significant benefits to both the health and wellbeing of patients. Because the question asks about both technology and remote care, we have answered ‘not sure’ in the context of risk linked to clinical delivery. All of the risks to patients set out in our response to earlier questions are amplified by this mechanism of delivery. However, at this point we cannot be certain whether or not these risks will impact patient safety.
The recent acceleration in remote care and the delivery of care using AI technologies has provided more optometrists with experience in delivering care in this way. This has provided examples of where patient risk is located, and we have included some of these examples below.
The evidence-base for some of the new technologies used in optometry is unlikely to currently exist due to the newness of the technology and the novel ways in which such new technologies can be used.
The main aspect of remote care where we have concerns is the risk of separating clinical procedures by time and place, across remote locations, where this isn’t done in interests of patients and where there is a reduction in clinical oversight. With regard to AI, we are concerned that there is a risk of deskilling the profession and also the risk of bias that may be present in the reference database for the technology.
Optometrists need to consider the whole clinical picture to protect against missed pathology and missed co-morbidities and must remain in control of clinical decision making. There is a risk that co-morbidities could be missed when the technology has a narrow focus and is only looking for one condition with a particular test or piece of software.
Evidence of risk – member feedback
Images
- “Photos taken by patients using mobile devices are often not clear enough. Smart phones can give a mirror view (be reversed), so the opposite eye shown in the photo of the patient says RE a problem camera photo shows its LE. I also often find the redness seen in the photo is less on the camera phone image compared to when examined face to face. This is caused by how the camera automatically balances the colour of the image. This can lead to inaccurate decision making if not carefully and appropriately overseen”
- “Where a patient is looking off centre when the scan is being taken, the disc map (a test to look for glaucoma) will reveal that the values are outside of the normative database values. This does not mean that the patients optic nerve head is unhealthy – it simply means the scan needs to be repeated and reassessed”
- “The normative database for people under the age of 20 is minimal and therefore, the results of the scan do not give the same amount of data as for someone 40+, this could produce spurious results without a clinician to oversee this”
Clinicians must be allowed extra time to evaluate any scans in detail to that they can avoid errors. While there is an opportunity to increase productivity, this must be tempered against the risk of errors such as viewing the wrong images or scans. There is also a requirement to ensure sufficient time is allowed for the scan to be taken, while recognising that the patients most likely to require additional tests may also be those that find them the hardest to complete.
Remote examination
Our view is that there is a risk that by stripping an examination down to it constituent parts it is far less than the whole; therefore, it is important that the clinician remains in charge of the problem-orientated examination, as with the following examples from member feedback:
- “A diabetic patient who had rung an optometrist saying their vison was blurred was told they were ok to leave as they had a diabetic retinopathy screening within one week. When I saw the patient face to face, his head turn showed obvious left lateral rectus palsy. The false reassurance that the diabetic system would pick up the problem placed this patient at risk of harm”
- “The patient reported ‘fuzzy vision’, but this turned out to be a bitemporal hemianopia when examined. The VA test in isolation showed 6/5 R +L, but on full examination the problem was found”
- “A post cataract patient was told by the hospital eye service that they had astigmatism, that it was this that was causing the blurred vision and that once they had spectacles it would solve the problem. Thankfully, the patient rang the GP who heard them mention distortion or a “curtain” and advised a face-to-face MECS, at which they were found to have a detachment”
There are also numerous examples of patients mentioning something seemingly unrelated, but when put together with other clinical tests a more serious problem is found, such as a simple sub conjunctival haemorrhage, linked to malaise and retinal signs leading to a diagnosis of leukaemia.
Practitioner wellbeing
Practitioner wellbeing is threatened with the push to increase productivity by utilising remote care, as more patients are seen and less time per patient is provided. There is already a culture in some parts of the sector for: optometrists to be given less time to care for patients due to financial incentives that encourage optometrists to see more patients; undue pressure on employees to see more patients with less time to interrogate test results.
Our recent AOP ‘Managing risk in practice’ advice was produced to acknowledge this problem. This trend is likely to become more stark with an increasing reliance upon remote care, with optical professionals expected to examine larger numbers of patients in a short time period. This poses a risk to practitioner mental and physical wellbeing and could risk patient safety.
Q47. Are there any unnecessary regulatory barriers in the Act that would prevent any current or future technological development in the eye care sector or restrict innovative care delivery or competition in the market?
AOP response: No
There are no regulatory barriers that prevent the use of optometrist-led technology. While the Act contains appropriate patient protections, in our opinion these do not prevent technological innovation. However, given the rapid pace of advancement, this is an area where the GOC needs to monitor emerging risks to ensure that those who seek innovation do not exploit loopholes which may place patients at risk.
The GOC’s view taken in the consultation document that ‘we should not make things difficult for people in the market or for those who wish to enter the market’ is in our opinion not the right approach. Imposing regulations sometimes does make the market more complex or difficult for new entrants but that this should not be used as a reason to avoid regulation. It is important to place patient protection at the heart of all decisions, many innovators or disruptors subsequently introduce risk to the market. There are well documented examples from other sectors, such as Airbnb and Uber.
Data and research
Information collected by AI systems can enhance our understanding of clinical population norms. This information could benefit national populations, enhance clinical research opportunities, and influence health funding decisions. But who owns this data and how will this data be used for public benefit and research? There is also the risk that the data obtained may introduce bias or underrepresent those with greatest need. There is an established link between ethnicity and socio-economic status, but there is also an established link between eye disease and ethnicity. If those at greatest risk are not correctly represented within the technology, they may be poorly served by the technology. This could widen health inequalities. Numerous studies have identified the presence of racial and other forms of biases within the datasets used by healthcare analytics and intelligence systems, and which describe the problems they pose for the use of algorithms and AI systems in healthcare.39,40, 41
References
39. Ghassemi, M., Oakden-Rayner, L., & Beam, A. L. (2021). The false hope of current approaches to explainable artificial intelligence in health care. The Lancet Digital Health, 3(11), e745-e750
40. Panch, T., Mattie, H., & Atun, R. (2019). Artificial intelligence and algorithmic bias: implications for health systems. Journal of global health, 9(2)
41. Rajpurkar, P., Chen, E., Banerjee, O., & Topol, E. J. (2022). AI in health and medicine. Nature Medicine, 28(1), 31-38
Q48. Are there any gaps within the Act or GOC policy relating to the regulation of technology or remote care that present a risk to patients?
AOP response: Yes
We are concerned that the GOC has not evolved its regulatory approach to meet these challenges and protect patients from companies offering components of online/remote optical care without appropriate registrant involvement and/or standards of care. Regulatory oversight needs to adapt to maintain public protection.
We note with interest (and support) that the Medicines and Healthcare products Regulatory Agency (MHRA) has announced plans to strengthen regulation of medical devices including medical devices that involve AI.
Appropriate regulatory mechanisms should be used to manage the risks that arise from new technologies, such as AI and remote care. This includes standards for individuals and businesses, including GOC regulatory action where care is being delivered in novel ways that pose risks to patients.
We outline the risks of separating clinical procedures by time and place, across remote locations where this is not done in the interests of patients and/or where it reduces clinical oversight of care in our answer to question 46 above.
Accurate machine refraction is already available, but there is a current protection to ensure that it is delivered by trained professionals. There will be a transition period when we will need a regulatory framework as technology and in particular AI becomes more widespread. This may include, but would not be limited to, complete regulatory clarity about who is responsible for the decision that the AI takes. Registrants, businesses, manufacturers, and patients need to understand who is ultimately responsible for the decision made by AI technology: is the AI merely a guide to a decision made by an individual clinician, or is the business who has purchased the technology or the manufacturer responsible for its decisions? Each approach brings differing regulatory challenges. How this environment develops in the future and how the new technologies are managed within a clinical environment are unknowns.
The majority of eye care is currently delivered by GOC registrants. As AI and technology use grows more important in eye care services, the approach to regulation of activity and individuals will need to become closer to that for the regulation of medical devices. And if the scope of primary eye care widens to include more involvement with eye care than is currently provided in a hospital setting, for example, it will become more important to ensure that the approach to the regulation of optometrists dovetails more closely with the related professions such as ophthalmology.
Part of the Health and Care Act changes make it a requirement that all organisations within the health sector must have a leading group to ensure the technology is safe. At Moorfields for example, a clinical safety group is being set up. Manufacturers have a level of responsibility, but the safety group has responsibility for ensuring the technology being used is clinically safe.
Patient interest needs to be at the centre of any technological developments and registrants need to be involved in these developments. We agree that regulation needs to reflect the dynamic nature of this space, but we believe that regulation of AI currently lags behind other emerging technological developments.
Our members comments have focused on concerns around responsibility and accountability and a want to see a clear demarcation of who is responsible when eye healthcare is delivered in novel ways.
There are other risks for the GOC to consider. In AI technologies there is a risk of bias in the data collected as the data collected is more likely to emanate from a small section of the population. As OCT scans come at a cost to the patient, it is likely that those most likely to opt for this additional service will be the more affluent patients.
The resulting data, which shows incidences of eye disease in just this particular set of patients, could result in resources (future research) skewed towards the most affluent.
Q49. If you answered yes to the previous question, do you have any suggestions about how these gaps in the regulation of technology or remote care could be addressed?
Regulation should ensure that appropriately skilled optical professionals remain responsible for decision making around eye care, and that their role for patient care is not adversely affected by the use of new technology or remote care.
Good regulation in this sphere would help in managing the implications of new technology. The growth of the use of artificial intelligence to support diagnostic decision-making and screening raises questions about the interaction between regulation of individual practitioners and the regulation of devices. The increasing delivery of services and sales of medical devices via the internet is challenging the traditional limits of regulation. Regulation must evolve to maintain public protection.
Our view is that we need to respond to gaps in an agile way. New risks that emerge will require the regulatory approach to evolve to the novel ways in which new technologies will be used. How the GOC documents all emerging risks within the sector is something the GOC has said it will incorporate in its review cycle. How technology and remote care is developed and managed in the future is a key emerging risk.
Whilst there are no specific questions about the optometrist workforce and their knowledge and skills, we want to make the following points:
- Planning and provision towards the future needs to enable the necessary shift in workforce culture
- Enhancing the role of the optometrist in interpreting and conveying the results of complex tests and information to patients in appropriate language, including imparting worrying news, human interaction with patients (relaying the prognosis to patients) all requires emotional intelligence. Especially so with patients with more complex needs -children, those with learning disabilities, and the elderly
- Data skills, knowledge of how AI technologies work, the ethics of data collection and data sharing are all skills which will become increasingly important for optometrists to have so that they can deliver the best possible care for patients
Q50. Are there any gaps in the Act or GOC policy relating to the regulation of online sales of optical appliances that present a risk to patients?
AOP response: Yes
We recognise that regulation of eye care and optical appliances from outside the UK is challenging, but we believe it is an area that the GOC should improve its regulatory approach towards in order to manage the evolving risks of harm to patients. These risks to patients arise from two sources:
- Overseas sellers of optical appliances, especially contact lenses and optical appliances marketed to under-16s
- Delivery of eye care services, consultations and tests
The increasing availability of optical appliances on online marketplaces and websites represents the most significant current risk of harm to patients, and this risk is growing as consumer behaviour switches to online purchasing habits, where it is harder to identify the seller’s country of origin or compliance with regulation.
Risks of harm from overseas online sales
There are a range of risks of harm to patients including infection, eye disease and sight loss that are associated with the supply of optical appliances outside of current restrictions, as we have explained in our answer to Section 6. A recent study42 about the habits of patients who purchase their contact lenses solely online showed that there was an increased likelihood in this group of initiating contact lens wear without any contact with eye care professionals. This demonstrates how sales from outside the UK, where sellers do not comply with UK regulations, could erode patient engagement with eye care services and put their eye health at risk. The main areas of risk are:
- Sales of contact lenses, where there is no check on whether the patient has had a recent fitting or sight test – increasing the risks of poor contact lens care leading to infection which can lead to sight loss
- Sales of optical appliances to under-16s where there is no supervision from an optical professional – leading to risks of poor visual fit and sight loss where development issues such as amblyopia are not identified
- Changing consumer behaviour leading to patients attending less frequently for sight tests – leading to reduced opportunities for detection of early-stage asymptomatic eye disease such as glaucoma
Increasing (and effectively unregulated) online supply could also affect the optical sector and professional practice in ways that could create further risks of public harm. For instance, our members are concerned that growing levels of online illegal sales could lead some practices to move out of contact lens care provision. This is because it will become uneconomic to provide contact lens services if patients have a contact lens fitting in the practice and then go online to source contact lenses from suppliers which, while cheaper, don’t comply with the UK legal requirements.
Emerging threats from overseas eye care services
Emerging technology will make it increasingly feasible to offer elements of a sight test such as refraction online. This could pose new risks to patients by reducing the role of clinicians and undermining the role of the sight test in identifying eye disease, lead to missed diagnoses and misdiagnoses. We have recently raised concerns with the GOC about the implications of an online supplier offering patients an initial refraction which is only followed by an eye health check if they order and collect an optical appliance.
Remote care carries risks of harm if it is not delivered in a way that is in the interests of the patient or diminishes optical professionals’ control over clinical decision making. As technology evolves it will become increasingly possible for overseas providers to market eye care services, including tests, diagnostics and consultative advice to patients remotely from outside the UK. The main risks here are that overseas providers could operate in ways that are not in accordance with UK optical regulations – using non-GOC registered professionals who are poorly trained, use tests that lack validity and do not understand the issues that apply in UK healthcare.
The Europe Economics risks assessment, commissioned by the GOC in 201443 to identify the costs of illegal practice, concluded that “misdiagnosis of diseases has both a high severity of an adverse event, and a medium-high likelihood of an adverse event occurring under illegal practice”.
References
42. Mingo-Botín, D., Zamora, J., Arnalich-Montiel, F., & Muñoz-Negrete, F. J. (2020). Characteristics, behaviors, and awareness of contact lens wearers purchasing lenses over the internet. Eye & Contact Lens, 46(4), 208-213
43. Europe Economics report: health risk assessment for illegal optical practice (2014), GOC website
Q51. If you answered yes to the previous question, do you have any suggestions about how these gaps in the regulation of online sales of optical appliances could be addressed?
We have made several suggestions to the GOC about how it can improve its regulatory approach to managing the risks of illegal online sales from outside the UK in recent years, including in 2021 those set out in our response to the GOC stakeholder survey on illegal practice and in response to its new illegal practice strategy. We welcomed small improvements in its revised illegal practice protocol, which was approved by the GOC council on the 1 July 2022, and now includes provision for contacting online marketplaces, such as Amazon, to remove listings that do not comply with UK regulations – but there is still much more that the GOC needs to do in order protect patients from risks of harm.
In summary the GOC approach to tackling illegal online sales and services from non-UK sellers and providers should include:
- Contacting the overseas seller to inform them about UK optical regulations and encourage compliance
- Contacting enforcement bodies based in the seller’s country
- Working with the sector to develop a mechanism (such as a kitemark) that can assist the public identify when they are buying optical appliances from a seller that is complying with UK regulations
- Educating the public about the risks of buying optical appliances from online sellers who do not operate within UK optical regulations
- Continuing to work with other healthcare regulators to identify strategies to deal with the risks of harm that arise from overseas healthcare products and services
- Monitor developments in UK legislation relating to online supply of products and services from outside the UK, which could present new pathways for GOC regulatory action against overseas illegal optical practice
Section 8: Any other areas
Q52. Are there other areas of our current legislation that you think need to be amended (recognising that the Department of Health and Social Care review will cover our core functions)?
AOP response: No
Q53. Are they any other gaps in regulation where you think legislative change might be required?
AOP response: Yes
Unsafe supply of optical appliances from sellers outside the UK, who do not comply with UK law, is a major and increasing source of risk for the public as we have explained in our answer to questions 50 and 51. The GOC should consider whether further legislative amendments are needed in order for it to better manage these risks of harm and protect the public. Alternatively, the GOC should consider if it can work more closely with other bodies to address this concern.
Q54. Are there any other policies or guidance that the GOC currently produces that should be reviewed or require amendments?
AOP response: Yes
Problems in GOC development of fitness to practise (FtP) allegations
Based on our representation of AOP members in FtP cases, we have ongoing concerns about the appropriateness and quality of the GOC’s framing of allegations in FtP cases. Accurate and properly constructed allegations, based on case information, are an important part of a well-functioning FtP process. Poorly framed allegations can lead to delays, costs and additional stress for those involved in the case. The GOC should improve its processes, policies and training relating to this aspect of the FtP pathway to ensure that the allegations it develops for cases are of a sufficient quality and accuracy.
Declarations Guidance
We regularly receive enquiries from our members regarding their obligation to declare health issues, criminal convictions, and disciplinary action from other regulators. We would welcome a review of the information currently provided to registrants regarding health declarations, particularly how they should proceed if they have an extended absence from work due to sickness but are expected to make a full recovery. We have previously offered to help in the development of any future policy and guidance and continue to make that offer.
Q55. Are there any other impacts of our legislation that you would like to tell us about, including financial impact or impact on those with protected characteristics under the Equality Act 2010 (ie age, sex, race, religion or belief, disability, sexual orientation, gender reassignment, pregnancy or maternity, caring responsibilities)?
AOP response: Yes
The Equality Act 2010 makes it against the law to discriminate against someone across nine protected characteristics including age, disability, gender reassignment, race, religion or belief pregnancy and maternity.
In this response we have talked much about the need to protect the most vulnerable and that we mitigate risk through safe, accessible high quality optometric care. It is our opinion that regulation and associated guidance should be predicated on ensuring equality of access and that any patient is not disadvantaged because of a protected characteristic or at any point over their life course.
Some patients with a protected characteristic are more susceptible/at risk of eye disease or sight loss for reasons including:
- Eye disease is more prevalent in some groups, for example Asian and Black ethnic groups are at greater risk of eye disease such as glaucoma and diabetic retinopathy44
- Contributing factors, for example pregnancy or fertility treatment can cause blurred vision or cause refractive change45
- Some people can be excluded or disadvantaged from accessing healthcare, for example black communities in the UK are less likely to attend primary eye care appointments despite the increased risk of sight loss46
References
44. Scase MO, & Johnson MR. Visual Impairment in ethnic minorities in the UK
46. Elam AR, & Lee PP. High-risk populations for vision loss and eye care underutilisation: A review of the literature and ideas on moving forward. 2013

